Six anodising FAQs
It’s a versatile process which is commonly used for protecting the surface of a range of metals, as well as making them look good. Indeed, as a treatment, it’s hard to beat. Read on to discover six FAQs about this interesting technique.
1. How is it achieved?
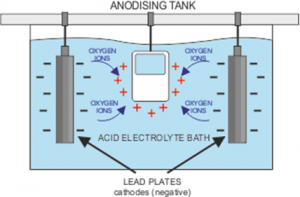
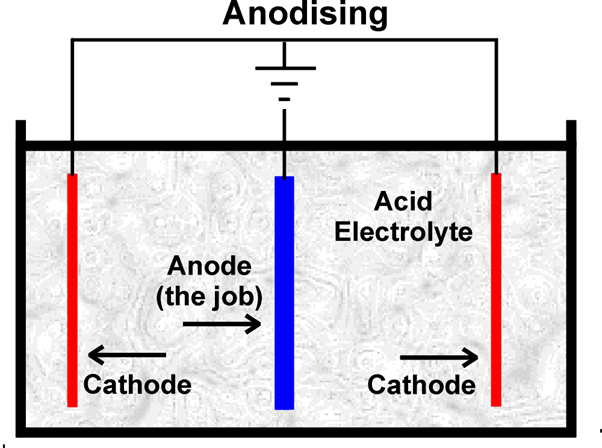
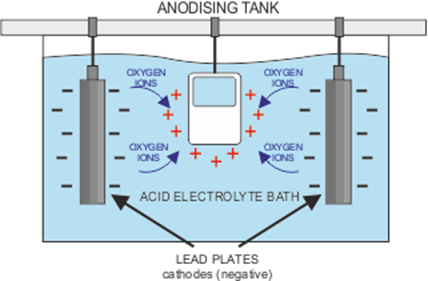
First of all, the chosen metal must be thoroughly cleaned and rinsed off, before being placed in a bath containing an electrolyte solution. The electrolyte is an electrical conductor containing positive and negative ions. The metal piece becomes an anode when a positive electrical current is applied, while the plates in the solution receive a negative charge. This makes the electrical circuit whereby the positive ions exchange places with the negative ions. For a full explanation, visit https://www.open.edu/openlearn/science-maths-technology/engineering-technology/manupedia/anodising.
2. What is a barrier layer?
You may come across the term ‘barrier layer’ and wonder what it means for the process. The anodisation process causes pores to develop on the surface of the metal as some of the positive ions escape. The longer the charge is applied, the deeper the pores extend. For aluminium, a layer of aluminium oxide which forms when the aluminium on the surface joins with the negatively charged Oxygen ions, and results in a protective shield against any chemical damage to the metal.
Image Credit
3. Which metals can be anodised?
As mentioned above, aluminium is a common candidate for anodisation. However, the treatment can also be applied to a wide range of other metals, such as titanium and magnesium, and even to certain plastics which have conductive properties. A beautiful and hard-wearing finish can be achieved on all of these materials, all at very little expense. This makes it the perfect choice for a range of uses, from body piercing jewellery through to architectural fittings. Discover other exciting new applications of this technology in this report https://www.theengineer.co.uk/technical-qa-advanced-materials/
4. What is hard anodisation?
Also referred to as Type III anodisation, this process offers the greatest defence against corrosion and extreme weathering. It is achieved by applying the electrical current to the solution for a longer period. The longer the current is applied, the deeper the depth of the aluminium oxide protection, up to 25 microns or greater. For more information, see anodising
5. How is colouration achieved?
One of the best features of the process is the ability to create attractive colour effects. This is achieved by adding pigments which fill the empty pores, meaning that the colour cannot then wear off or be scratched away.
6. Can a whole part be anodised?
A section of each part will remain unanodised, as it must be gripped when lowered into the electrolyte bath. Therefore it’s wise to add a handle which won’t need to undergo the process.
It’s clear that for a cost-effective reliable means of protecting metals and other materials, anodisation is the best option. Its versatility makes it an excellent choice for a range of applications and it looks attractive too. Why not explore how this process could work for you?
Leave a reply